Журнал «Здоровье ребенка» Том 17, №3, 2022
Вернуться к номеру
Патогенетична роль деяких цитокінів у розвитку та перебігу різних клінічних форм інфекцій сечовидільної системи в дітей
Авторы: H.O. Lezhenko, N.A. Zakharchenko
Zaporizhzhia State Medical University, Zaporizhzhia, Ukraine
Рубрики: Педиатрия/Неонатология
Разделы: Клинические исследования
Версия для печати
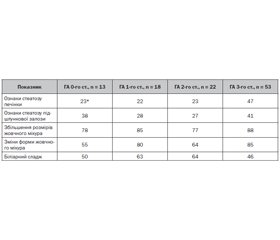
Мета роботи. Дослідити вміст цитокінів інтерлейкіну-6 та інтерлейкіну-15 у сироватці крові дітей з інфекцією сечовидільної системи та встановити їх патогенетичну роль у розвитку різних клінічних форм хвороби. Матеріали та методи. Групи дослідження становили 84 дитини (середній вік — 10,0 ± 1,3 року). Основну групу розділили на підгрупи: 1-ша — 17 дітей, хворих на гострий пієлонефрит; 2-га — 21 пацієнт із хронічним пієлонефритом; 3-тя — 16 дітей, хворих на гострий цистит; 4-та — 10 пацієнтів із неуточненими інфекціями сечовидільної системи. Контрольну групу становили 20 умовно здорових дітей. Методом імуноферментного аналізу досліджено вміст інтерлейкіну-6 та інтерлейкіну-15. Результати. Установлено, що розвиток гострих інфекцій сечовивідних шляхів супроводжувався підвищенням рівня сироваткового прозапального інтерлейкіну-6. Найвищим він був у дітей, хворих на цистит, — у 2,8 разa більше за показники контрольної групи (р < 0,01). У пацієнтів із гострим пієлонефритом уміст цитокіну було перевищено в 1,8 разa (р < 0,05). Однак у хворих на хронічний пієлонефрит ми спостерігали лише тенденцію до його зростання (р > 0,05). Рівень інтерлейкіну-15 в основній групі був статистично вищим за показники контрольної групи (р < 0,05). У дітей 3-ї та 4-ї підгруп його вміст не відрізнявся від такого в групі контролю (р > 0,05). Проте в 1-й (р < 0,05) та 2-й (р < 0,01) підгрупах ми спостерігали статистично значуще підвищення рівня інтерлейкіну-15. Також встановлено наявність прямої кореляційної залежності вмісту інтерлейкіну-15 від тривалості захворювання (r = 0,64, р < 0,05). Висновки. Розвиток гострого запального процесу в сечовивідних шляхах у дітей відбувається на тлі вираженого зростання експресії інтерлейкіну-6, тоді як хронічний запальний процес перебігає із статистично значущим підвищенням рівня інтерлейкіну-15 у сироватці крові.
Background. The purpose of the research: to study the content of interleukin-6 and interleukin-15 cytokines in the blood serum of children with urinary tract infection and to establish their pathogenetic role in the development of various clinical forms of the disease. Materials and methods. The study groups consisted of 84 children (mean age of 10.0 ± 1.3 years). The main group was divided into subgroups: the first one — 17 children with acute pyelonephritis, the second — 21 patients with chronic pyelonephritis, the third — 16 children with acute cystitis, the fourth subgroup — 10 patients with unspecified urinary tract infections. The control group included 20 relatively healthy children. The content of interleukin-6 and interleukin-15 was evaluated by enzyme-linked immunosorbent assay. Results. It was established that the development of acute urinary tract infections was accompanied by a high level of serum pro-inflammatory interleukin-6. We found the highest level in children with cystitis, which exceeded that of the control group by 2.8 times (р < 0.01). In children with acute pyelonephritis, this cytokine was 1.8 times higher (р < 0.05). However, patients with chronic pyelonephritis had only a tendency towards its increase (p > 0.05). Interleukin-15 in the main group was statistically higher than in controls (р < 0.05). In children of subgroups 3 and 4, its level did not differ from that of the control group (p > 0.05). However, in subgroups 1 (р < 0.05) and 2 (р < 0.01), we observed a statistically significant increase in interleukin-15 level. A direct correlation between interleukin-15 content and disease duration (r = 0.64, р < 0.05) was also found. Conclusions. The development of an acute inflammatory process in the urinary tract in children occurs against the background of a marked increase in the expression of interleukin-6, while a chronic inflammatory process develops with a statistically significant increase in the level of interleukin-15 in blood serum.
діти; інфекція сечовидільної системи; цитокіни
children; infection of the urinary system; cytokines
Introduction
Materials and methods
Results
Discussion
Conclusions
- Abraham S., Miao Y. The nature of immune responses to urinary tract infections. Nature Reviews Immunology. 2015. 15(10). 655-663. doi: 10.1038/nri3887.
- Al Rushood M., Al-Eisa A., Al-Attiyah R. Serum and urine interleukin-6 and interleukin-8 levels do not differentiate acute pyelonephritis from lower urinary tract infections in children. Journal of Inflammation Research. 2020. 13. 789-797.
- Balighian E., Burke M. Urinary tract infections in children. Pediatrics in Review. 2018. 39(1). 3-12. doi: 10.1542/pir.2017-0007.
- Berger C., Berger M., Hackman R., Gough M., Elliott C., Jensen M., Riddell S. Safety and immunologic effects of IL-15 administration in nonhuman primates. Blood. 2009. 114(12). 2417-2426.
- Ching C., Gupta S., Li B., Cortado H., Mayne N., Jackson A., McHugh K., Becknell B. Interleukin-6/Stat3 signaling has an essential role in the host antimicrobial response to urinary tract infection. Kidney International. 2018. 93(6). 1320-1329. doi: 10.1016/j.kint.2017.12.006.
- Devocelle A., Lecru L., François H., Desterke C., Gallerne C., Eid P., Estelle O., Azzarone B., Giron-Michel J. Inhibition of TGF-β1 signaling by IL-15: a novel role for IL-15 in the control of renal epithelial-mesenchymal transition: IL-15 counteracts TGF-β1-induced EMT in renal fibrosis. International Journal of Cell Biology. 2019. 2019. 1-15.
- Engelsöy U., Rangel I., Demirel I. Impact of proinflammatory cytokines on the virulence of uropathogenic Escherichia coli. Frontiers in Microbiology. 2019. 10. doi: 10.3389/fmicb.2019.01051.
- Fiore P., Di Matteo S., Tumino N., Mariotti F., Pietra G., Ottonello S., Negrini S., Bottazzi B., Moretta L., Mortier E., Azzarone B. Interleukin-15 and cancer: some solved and many unsolved questions. Journal for ImmunoTherapy of Cancer. 2020. 8(2). e001428. doi: 10.1136/jitc-2020-001428.
- Giron-Michel J., Azzi S., Ferrini S., Chouaib S., Camussi G., Eid P., Azzarone B. Interleukin-15 is a major regulator of the cell-microenvironment interactions in human renal homeostasis. Cytokine & Growth Factor Reviews. 2013. 24(1). 13-22.
- Hoen L., Bogaert G., Radmayr C., Dogan H., Nijman R., Quaedackers J., Rawashdeh Y., Silay M., Tekgul S., Bhat N., Stein R. Update of the EAU/ESPU guidelines on urinary tract infections in children. European Urology. 2021. 79. S446-S448.
- Leung A., Wong A., Leung A., Hon K. Urinary tract infection in children. Recent Patents on Inflammation & Allergy Drug Discovery. 2019. 13(1). 2-18. doi: 10.2174/1872213X13666181228154940.
- Masajtis-Zagajewska A., Nowicki M. New markers of urinary tract infection. Clinica Chimica Acta. 2017. 471. 286-291. doi: 10.1016/j.cca.2017.06.003.
- Mazaheri M. Serum interleukin-6 and interleukin-8 are sensitive markers for early detection of pyelonephritis and its prevention to progression to chronic kidney disease. International Journal of Preventive Medicine. 2021. 12(1). 2. doi: 10.4103/ijpvm.IJPVM_50_19.
- McInnes I., Leung B., Sturrock R., Field M., Liew F. Interleukin-15 mediates T cell-dependent regulation of tumor necrosis factor-α production in rheumatoid arthritis. Nature Medicine. 1997. 3(2). 189-195.
- Patidar M., Yadav N., Dalai S. Interleukin 15: a key cytokine for immunotherapy. Cytokine & Growth Factor Reviews. 2016. 31. 49-59. doi: 10.1016/j.cytogfr.2016.06.001.
- Rose-John S. Interleukin-6 family cytokines. Cold Spring Harbor Perspectives in Biology. 2017. 10(2). a028415. doi: 10.1101/cshperspect.a028415.
- Schluns K., Lefrançois L. Cytokine control of memory T-cell development and survival. Nature Reviews Immunology. 2003. 3(4). 269-279.
- Simões e Silva A., Oliveira E., Mak R. Urinary tract infections in pediatrics: an overview. Journal de Pediatria. 2020. 96. 65-79. doi: 10.1016/j.jped.2019.10.006.
- Spencer J., Schwaderer A., Becknell B., Watson J., Hains D. The innate immune response during urinary tract infection and pyelonephritis. Pediatric Nephrology. 2013. 29(7). 1139-1149. doi: 10.1007/s00467-013-2513-9.
- Steel J., Waldmann T., Morris J. Interleukin-15 biology and its therapeutic implications in cancer. Trends in Pharmacological Sciences. 2012. 33(1). 35-41.
- Su H., Lei C., Zhang C. Interleukin-6 signaling pathway and its role in kidney disease: an update. Frontiers in Immunology. 2017. 8. doi: 10.3389/fimmu.2017.00405.
- Tramma D., Hatzistylianou M., Gerasimou G., Lafazanis V. Interleukin-6 and interleukin-8 levels in the urine of children with renal scarring. Pediatric Nephrology. 2012. 27(9). 1525-1530. doi: 10.1007/s00467-012-2156-2.
- Vazouras K., Velali K., Tassiou I., Anastasiou-Katsiardani A., Athanasopoulou K., Barbouni A., Jackson C., Folgori L., Zaoutis T., Basmaci R., Hsia Y. Antibiotic treatment and antimicrobial resistance in children with urinary tract infections. Journal of Global Antimicrobial Resistance. 2020. 20. 4-10. doi: 10.1016/j.jgar.2019.06.016.
- Waldmann T., Tagaya Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annual Review of Immuno–logy. 1999. 17(1). 19-49.
- Ward P. The curiosity of IL-15. Nature Medicine. 2007. 13(8). 903-904.
- Zakon.rada.gov.ua. Treatment protocol for children with urinary tract infections and tubolointerstitial nephritis. Available from: https://zakon.rada.gov.ua/rada/show/v0627282-08#Text [in Ukrainian].

/14.jpg)