Introduction
For cancer and biological research, the Hela cell line is the first human cell line established through in vitro culture, and since 1952, this cell line has become the most widely studied human cell line in biology [1]. Hela cells are cervical cancer cells derived from Henrietta Lacks, an African-American woman who died of cancer in 1951. Apoptosis is disabled in these cells so they do not age to death and can multiply and divide indefinitely, growing rapidly [2]. Hela cells are adhering growing cells and the most common method for their cell culturing is the use of plastic petri dish cultures. After a certain period of culturing time, Hela cells attach to the bottom of the petri dish for proliferation and growth.
Nomura et al. previously reported using nanosheets as a new culture dish for Hela cells, combining nanotechnology with the field of biotechnology, (unnecessary coma) and found that the cell proliferation pattern on the surface nanosheets was the same as in the traditional culture method, but the cell adhesion pattern was different [3]. Data showed that nanomaterials can control the attachment of cells and have the ability to alter tissue growth.
In this context, carbon nanotubes have been explored as a nanomaterial based scaffold [4]. Carbon nanotubes (CNTs) are an allotrope of carbon and consist of hollow cylinders of wrapped graphene sheaths with a length of up to several microns and a diameter of several nanometers [5]. Since CNTs were first discovered as a new type of artificial carbon by Iijima in 1991 [6], they have been widely used in industrial products, nanoelectronics, biomedicine and other fields due to their unique physical and chemical propertie [7, 8]. A sub-class of CNTs are multi-walled carbon nanotubes (MWCNTs), which feature several graphene sheet layers and can also exhibit bamboo-like ultra-structure [7]. CNTs have unique mechanical properties, electrical properties and chemical stability [8]. Because of this special combination of properties, CNTs have been used as biosensors, DNA assembly scaffolds and substrates for cell growth and adhesion in tissue engineering [9–12]. For cell adhesion in tissue engineering, the compatibility and non-toxicity of CNTs to biological tissues should be considered. So far, non-toxic studies of carbon nanotubes have been controversial. For example, Magrez et al. found that CNTs are toxic to cells, while MWCNTs have the least toxicity in nanofibers and nanoparticles [13]. Kurantowicz et al. pointed out that carbon structures (graphite, graphene oxide, and diamond) are highly biocompatible and non-toxic to biological tissues [14]. Carbon nanotubes can be applied to the study of cell adhesion, in which the CNTs can be used as scaffolds to interact with cells and make cells adhere to the CNT scaffolds, thus affecting cell division [15].
It was shown that MWCNTs can shape three-dimensional scaffold structures, which is indispensable for maintaining cell growth and differentiation [16]. King et al. studied the relationship between scaffolds synthesized by the arrangement of CNTs and cartilage tissue, and the study showed that chondrocytes could grow effectively on carbon nanotubes, and carbon nanotubes had a morphological structure suitable for chondrocyte growth [17]. Holy et al. explored the adhesion, proliferation and differentiation of pluripotent stem cells on MWCNTs, and found that cell adhesion to the glass surface of multi-walled carbon nanotubes coating increased, and multi-walled carbon nanotubes would help guide the differentiation of pluripotent stem cells for tissue engineering purposes [11]. However, few reports have been reported on the interaction between multi-walled carbon nanotubes and Hela cells.
In this study, we use vertically aligned MWCNT scaffolds for in-vitro Hela cell culturing, and use electron microscopy to explore the growth, morphology and adhesion of the Hela cells to the nanostructured MWCNT scaffold versus a flat polished Si control surface. The Hela cells are generally elongated and spindle-shaped, but few studies have explored the morphological characteristics of Hela cells on corrugated surfaces. The Hela cell samples were chemically fixed to conduct electron microscopy, and the growth density and adhesion of the cells to the MWCNTs was characterized. The morphological characteristics of the Hela cells changed in different culture periods, which can be related to the division cycle of the Hela cells.
Materials and methods
Scaffold Synthesis
The MWCNT based nanostructured scaffold was produced by plasma-enhanced chemical vapor deposition (PECVD) to obtain vertically aligned MWCNTs on flat silicon surfaces [18].The growth follows the vapor-liquid-solid method (VLS), a common mechanism in one-dimensional nanostructure synthesis [19]. It employs liquid nickel droplets, which are used as a catalyst to form solid MWCNTs from decomposed acetylene (C2H2) gas admixed with ammonia (NH3) carrier gas [20]. The growth of MWCNTs by PECVD was thoroughly described by Ren et al. [21]. We used a 10 nm thick nickel film, which was deposited by electron beam evaporation, and dewetted this film into Ni nanodroplets at 800 °C. Vertical MWCNT growth results from the directional bombardment of positive ions onto the sample surface in the direct-current plasma (DCP), where the nickel catalysts particles protect the MWCNT body from this physical etching effect. This process is highly dependent on parameters including temperature, precursor gases and their ratio, system pressure, plasma power, catalyst material and growth time. In this study these parameters were 800 °C temperature, 20 W plasma power, employing 50 sccm C2H2 to 200 sccm NH3 gas flow, the pressure was 1kPa and the growth time was 30 minutes.
Cell Harvest and in vitro Culture
Hela cells grow in a nutritionally complete synthetic medium, consisting of 89 % DMEM (Hyclone, high glucose culture medium), 10 % inactivated fetal bovine serum (Hyclone), and 1 % penicillin-streptomycin double antibody (Macklin). First, the medium is pre-heated to 37 °C. After that 10 mL culture medium was added to the cell suspension and centrifuged for 5 min at 1000 rpm. After centrifugation, the supernatant was discarded and 1 mL culture medium was added before transferring the cell suspension to a culture flask with unsealed T25 port. A volume of 5 mL culture solution was added and gently shook to evenly mix the cell suspension. Cell culturing was conducted in an incubator containing 5 % CO2 at 37 °C and the medium was changed once a day during culturing.
During cell passage, the old medium was sucked out and trypsin/EDTA (Sigma) 2 mL was added for digestion and were observed under a microscope. When the cells become round, they were added into the medium twice as fast to neutralize them, so as to avoid excessive digestion and death of the cells. Then cell suspension was collected and placed in a centrifuge tube and centrifuged at 1000 rpm for 5 min. After centrifugation, the supernatant was removed and 1 mL of new culture medium was added for cell resuspended. For cell counting, 10 μL cell suspension and 10 μL Trypan blue dye (Gibco) were mixed 1 : 1, then 10 μL mixed liquid was dropped onto the cell counting plate, and the number of cells was calculated as 2.71 × 106 cells, and the cell survival rate was about 90 %.
The MWCNT scaffold and silicon wafers were disinfected with UV light for half an hour before being placed into a 60 mm petri dish. Finally, the cell suspension was transferred to the petri dish, and proper amount of fresh culture medium was added until the scaffold and the upper surface of the substrate were immersed, and placed into the incubator at 37 °C, 5 % CO2, 95 % air and 100 % humidity.
SEM Analysis of Cells Cultured on the MWNCTs-Based Scaffold
After 5 hours and 24 hours of culture time respectively, the petri dish was taken out, the culture medium was removed, and the MWCNT scaffold and silicon wafer surface were rinsed with PBS (Biosharp). To prepare the samples for scanning electron microscopy the Hela cells were chemically fixed [22]. The MWCNT scaffolds and silicon wafers were first immersed in 25 % glutaraldehyde fixative precooled to 5 °C (Damas-beta). After fixation at 5 degrees for 2 hours, the fixator was desorbed by adding and soaking them in 3 mL PBS for 10 min each, twice. The sample was then dehydrated by placing the sample sequentially through a series of ethanol to water solutions (30 %, 50 %, 70 %, 80 %, 90 %, 95 %, and 100 % volume by volume) for 15 minutes each. Hexamethyldisilazane (HMDS Aladdin) was to dry the sample. First, the sample were transferred from 100% ethanol to 1 : 2 HMDS : 100% ethanol solution and were soaked for 20 minutes. The samples were transferred to a fresh 2 : 1 HMDS : ethanol solution and soaked for another 20 min. Then the samples were transferred into 100% HMDS and were left to soak for 20 minutes. This was repeated and the samples remained in HMDS overnight. Finally, the HMDS was evaporated and the samples are ready for Scanning electron microscopy for imaging. The surface of the sample was characterized using a ZEISS Sigma 500 SEM, and the geometry of the MWNTS was characterized by image analysis software (Nano Measurer 1.2).
Results
Vertically Aligned MWCNT Scaffold growth
Vertically aligned MWCNTs were grown on polished silicon substrates and characterized by SEM as shown Fig. 1. Two different perspectives are shown, i.e. a 45° tilted view (Fig. 1A and B) and a top view Fig. 1C. From the tilted view, it is clear, that the MWCNTs are straight and aligned in parallel in respect to each other. Furthermore, the top view confirms the vertical alignment of the MWCNTs in respect to the Si substrate surface, which can be concluded from the MWCNT bodies being concealed by their apex. The average length of the MWCNTs is L = 1.591 µm, which we extracted from the tilted view images applying trigonometric considerations, and their average diameter D = 124 nm, which was obtained from Fig. 1C. Similar, the average nearest neighbor distance d = 152 nm was calculated using particle analysis software tools. The apex of the MWCNTs appears bright due to the nickel catalyst particles encapsulated at the MWCNT tips.
Hela Cell Culture on the Vertically Aligned MWCNTs Scaffold vs. polished Si
Hela cells were incubated for 5 and 24 hours on the vertically aligned MWCNT based scaffolds. At the same time, Hela cells were also incubated on polished Si substrated as the control group. After the incubation time, the cells were fixed for SEM imaging. We coated these biological sample with a 10 nm Au metal film to improve their electrical conductivity to avoid electrostatic charging artifacts during SEM imaging. Even though, this thin coating increases the thickness of very small features, the morphology of the cells can be observed without meaningful alterations. We observe the cultured Hela cells at different magnifications to comprehensively illustrate their growth and adhesion on the two distinct surfaces at different culturing times.
After culturing, we observe adhered cells on both surfaces shown in Fig. 2 at 300x magnification. Hela cells, which were cultivated for 5 h, are shown in Fig. 2A and B for the VA-MWCNT scaffold and the polished silicon substrate, respectively. The cells grown on the VA-MWCNT scaffold exhibit a mostly fusiform morphology indicated by arrow a, and some are slightly flattened, epithelioid cell as indicated by arrow b (Fig. 2A). In contrast, the cells grown on polished silicon are mostly of spherical morphology (Fig. 2B) as indicated by arrow c. The overall number of cells is comparable on both substrates with moderate density and free substrate area. When the cell culture time is extended to 24 h, it is evident that more cells grow on the surface of both surfaces. On the VA-MWCNT based scaffold the Hela cells feature have a round shape, can clearly be distinguished, and cover almost the entire surface as shown in Fig. 2C. However, the cells did not uniformly spread and grow on the polished silicon wafers, and the cells with spherical morphology that were observed after 24 h culturing became larger and of flattened fusiform morphology (Fig. 2D). Furthermore, these cells seem to be intertwined, making it impossible to distinguish individual cells. On both substrates, the overall size of the cells increases with culturing time and their shape changes from elongated spindle and spherical shapes towards flat spindle morphology.
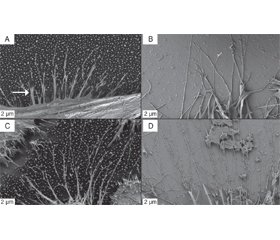
SEM images at 2 kx magnification reveal the detailed morphology of the individual Hela cells. As shown in Fig. 3, the Hela cells adhere differently on the surface of the two different substrates after 5 h culturing time. Filamentous pseudopodia (arrow) extended from the cell body and attached to individual VA-MWCNTs on the MWCNT based scaffold (Fig. 3A). These filamentous pseudopodia don’t spatially extend far from the cell body and entangle between the pseudopodia of neighboring cells. In contrast, the Hela cells cultured on polished silicon extend longer filamentous pseudopodia, which also entangle between neighboring cells (Fig. 3B). When the cell culture time was extended to 24 h, it was found that the morphology of the cells changed. The cells on the VA-MWCNT showed a round shape (Fig. 3C), and the cells on the polished silicon wafer were not only spherical, but also flattened (Fig. 3D). At this magnification, the more flattened cell morphology of the Hela cells on polished silicon vs. a more spherical cell morphology on VA-MWCNT based scaffolds is evident. The contours of the cell surface are very irregular, which is more pronounced for the Hela cells grown on polished silicon substrates.
When the magnification of the microscope was adjusted to 5 kx, the growth of the filopodiaprosthetic foot ends after 5 h and 24 h culture in Hela cells could be seen, as shown in Fig. 4. It can be seen that the pseudopodia pull on the tips of the –VA-MWCNTs immediately adjacent to the cell body (arrow) and some pseudopodia extend further outward along the tips of individual MWCNTs and sho–wing bifurcations at their ends (Fig. 4 A, C). The pseudopodia of the Hela cells grown on polished silicon are longer and slender featuring similar tapered and bifurcated ends (Fig. 4B, D). From this magnification, the filamentous pseudopodia of the cells did not change the way they clung to the base over time.
Discussion
Bright contrast nickel catalyst particles at the top of the MWCNTs could be seen in (Fig. 1C), resulting from the tip-growth tip of each MWCNTs. Previously it was indicated that despite the high nickel content in such scaffolds, there was no cytotoxic effect on chondrocytes, which may be due to the fact that nickel particles were encapsulated in MWCNTs, forming a barrier between nickel particles and chondrocytes [16]. This explains why these scaffold show no toxic effect to the Hela cells in this work.
/11.jpg)
The overall number of cells is comparable on both substrates with moderate density and free substrate area for short culture times of 5 h (Fig. 2A, B). After 24 h of cell culture, the number of cells on the MWCNTs site was significantly higher than that on the polished silicon wafer (Figure 2C, D), which indicates that the MWCNTs substrates are more favorable for the proliferation and growth of Hela cells. It is known that the cell surface morphology of Hela cell line was closely related to the DNA synthesis cycle in the nucleus [23]. The cell cycle is divided into intercellular phase and mitotic phase. DNA replication occurs during the intercellular phase (G1, S, G2), followed by mitosis. Attachment and diffusion usually occurs in the stages of the preparation for protein synthesis, protein synthesis and preparation for mitosis (G1, S or G2). It can be said that in the early stage of cell proliferation, cells may be spherical [24]. With the increase of culture time, cell proliferation and growth the cell volume changes, and the spherical shape gradually fades to a flake shape (Fig. 2C, D). Cells in synchronous culture had different morphologies in Fig. 2, indicating that not all cells grew synchronously and had the characteristic appearance of the same period. Under the same culture time, the morphology of Hela cells in different substrates was different. It can be seen from (Fig. 2A) and (Fig. 2B) that the cells on the base of MWCNTs were flattened earlier. It seems that the MWCNTs have a beneficial effect on the cell growth cycle, which might be one of the reasons for the larger number of cells growing on the substrate of MWCNTs at the same culture time. From (Fig. 3C, D), it was found that the individual cells on the substrate of MWCNTs were more easily distinguished, whereas the individual cells on the polished silicon wafers were intertwined. It may be that MWCNTs allow individual cells to cling to scaffolds instead of entangling with each other.
/12.jpg)
The cells are believed to adhere to the basal surface and grow due to the attachment of filopodia. However, it was evident that the filopodia of the cells on the surface of polished silicon wafers (Fig. 3B, D) extended longer than those on the basal surface of MWCNTs (Fig. 3A, C). It is possible that the presence of a scaffold allows filopodia to attach to a nearby scaffold and cells to grow easier on such a substrate. For polished silicon wafers without a scaffold, the filamentous pseudopodia of the cells may need to be clamped farther to allow the cells to grow closer to the substrate.
Conclusions
In this paper, the growth of Hela cells on the surface of silicon wafers containing MWCNTs was studied by scanning electron microscopy and compared with that of ordinary polished silicon wafers. It was shown that MWCNTs were useful for Hela cell growth. The number of cells growing on the surface of silicon wafers on MWCNTs was higher than that on the ordinary polished silicon wafer surfaces. Over the same culture time period, there were significant differences in cell morphology on these surfaces. The cell growth morphology is related to the stage of DNA replication. Hela cell globules may appear in the early G1 and mitotic stages of intercellular phase. The interaction between cell extension and carbon nanotubes led to changes in cell morphology and growth direction. MWCNTs may also play a role as a cell growth promoter, accelerating cell proliferation and division. This causes the cells to multiply and divide earlier than those grown on normal silicon wafers, resulting in a flattened state. Overall, MWCNTs provide a good scaffold for the growth of Hela cells. Furthermore, the interaction between carbon nanotubes and biological tissues is beneficial for fast cell growth.
Received 20.01.2022
Revised 02.02.2022
Accepted 09.02.2022
Список литературы
1. Landry J.J.M., Pyl P.T. et al. The Genomic and Transcriptomic Landscape of a HeLa Cell Line. G3 (Bethesda). 2013. 3(8). 1213-1224. https://doi.org/10.1534/g3.113.005777.
2. Lyapun. I.N., Andryukov. B.G., Bynina M.P. HeLa Cell Culture: Immortal Heritage of Henrietta Lacks. Mol. Genet. Microbiol. Virol. 2019. 34(4). 195-200. https://doi.org/10.3103/S0891416819040050.
3. Nomura S., Kojima H., Ohyabu Y., Kuwabara K., Miyauchi A., Uemura T. Cell Culture on Nanopillar Sheet: Study of HeLa Cells on Nanopillar Sheet. Jpn. J. Appl. Phys. 2005. 44(37). L1184-L1186. https://doi.org/10.1143/JJAP.44.L1184.
4. Firkowska I., Olek M., Pazos-Peréz N., Rojas-Chapana J., Giersig M. Highly Ordered MWNT-Based Matrixes: Topography at the Nanoscale Conceived for Tissue Engineering. Langmuir. 2006. 22(12). 5427-5434. https://doi.org/10.1021/la053067e.
5. Edwards S.L., Church J.S., Werkmeister J.A., Ramshaw J.A.M. Tubular Micro-Scale Multiwalled Carbon Nanotube-Based Scaffolds for Tissue Engineering. Biomaterials. 2009. 30(9). 1725-1731. https://doi.org/10.1016/j.biomaterials.2008.12.031.
6. Lijima S. Helical microtubules of graphitic carbon. Nature. 1991. 354(6348). 56-58. https://doi:10.1038/354056a0
7. Bottini M., Bruckner S., Nika K., Bottini N., Bellucci S., Magrini A., Bergamaschi A., Mustelin T. Multi-Walled Carbon Nanotubes Induce T Lymphocyte Apoptosis. Toxicol. Lett. 2006. 160(2). 121-126. https://doi.org/10.1016/j.toxlet.2005.06.020.
8. Zhang X., Wang X., Lu Q., Fu C. Influence of Carbon Nanotube Scaffolds on Human Cervical Carcinoma HeLa Cell Viability and Focal Adhesion Kinase Expression. Carbon. 2008. 46(3). 453-460. https://doi.org/10.1016/j.carbon.2007.12.015.
9. Correa-Duarte M.A., Wagner N., Rojas-Chapana J., Morsczeck C., Thie M., Giersig M. Fabrication and Biocompatibility of Carbon Nanotube-Based 3D Networks as Scaffolds for Cell Seeding and Growth. Nano Lett. 2004. 4(11). 2233-2236. https://doi.org/10.1021/nl048574f.
10. Abarrategi A., Gutierrez M.C., Moreno-Vicente C., Hortigüela M.J., Ramos V., Lopez-Lacomba J.L., Ferrer M.L., Del Monte F. Multiwall Carbon Nanotube Scaffolds for Tissue Engineering Purposes. Biomaterials. 2008. 29(1). 94-102. https://doi.org/10.1016/j.biomaterials.2007.09.021.
11. Holy J., Perkins E., Yu X. Adhesion, Proliferation and Differentiation of Pluripotent Stem Cells on Multi-Walled Carbon Nanotubes. IET Nanobiotechnol. 2011. 5(2). 41-46. https://doi.org/10.1049/iet-nbt.2010.0014.
12 Akinoglu E.M., Ozbilgin K., Kilicaslan Sonmez P., Ozkut M.M., Giersig M., Inan S., Gumustepe E., Kurtman C. Biocompatibility of vertically aligned multi-walled carbon nanotube scaffolds for human breast cancer cell line MDA-MB-231. Progress in Biomaterials. 2017. 6(4). 189-196. https://doi.org/10.1007/s40204-017-0078-6
13. Magrez A., Kasas S., Salicio V., Pasquier N., Seo J.W., Celio M., Catsicas S., Schwaller B., Forro L. Cellular Toxicity of Carbon-Based Nanomaterials. Nano Lett. 2006. 6. 1121-1125. https://doi.org/10.1021/nl060162e.
14. Kurantowicz N., Strojny B., Sawosz E., Jaworski S., Kutwin M., Grodzik M., Wierzbicki M., Lipińska L., Mitura K., Chwalibog A. Biodistribution of a High Dose of Diamond, Graphite, and Graphene Oxide Nanoparticles After Multiple Intraperitoneal Injections in Rats. Nanoscale Res. Lett. 2015. 10(1). 398. https://doi.org/10.1186/s11671-015-1107-9.
15. Sosnowska M., Sawosz E., Kutwin M., Chwalibog A. Carbon nanoscaffolds for fibroblast and hepatocellular carcinoma cells adhesion, migration and regeneration. Biomaterials. 2017. 143(20). 58.
16. Trzeciak T., Rybka J.D., Akinoglu E.M., Richter M., Kaczmarczyk J., Giersig M. In Vitro Evaluation of Carbon Nanotube-Based Scaffolds for Cartilage Tissue Engineering. J. Nanosci. Nanotechnol. 2016. 16(9). 9022-9025. https://doi.org/10.1166/jnn.2016.12733.
17. King A., MattaDomjan B., Large M.J., Matta C., Ogilvie S.P., Bardi N., Byrne H., Zakhidov A., Jurewicz I., Velliou E.G., Lewis R., LaRagione R., Dalton A.B. Pristine carbon nanotube scaffolds for the growth of chondrocytes. Journal of Materials Chemistry B. 2017. https://doi.org/10.1039/C7TB02065A.
18. Ren Z.F., Huang Z.P., Xu J.W., Wang J.H., Bush P., Siegal M.P., Provencio P.N. Synthesis of large arrays of well-aligned carbon nanotubes on glass. Science. 1988. 282(5391). 1105.
19. Wagner R.S., Ellis W.C. Vapor-liquid-solid mechanism of single crystal growth. Appl. Phys. Lett. 1964. 4(5). 89-90. https://doi.org/10.1063/1.1753975.
20. Meyyappan M., Delzeit L., Cassell A., Hash D. Carbon Nanotube Growth by PECVD: A Review. Plasma Sources Sci. Technol. 2003. 12(2). 205-216. https://doi.org/10.1088/0963-0252/12/2/312.
21. Ren, Z., Lan, Y., Wang, Y. Aligned carbon nanotubes: physics, concepts, fabrication and devices. NanoSci. Technol. 2013. https://doi.org/10.1007/978-3-642-30490-3_3.
22. Kashi A.M., Tahermanesh K., Chaichian S., Joghataei M.T., Tavangar S.M., Najafabadi, A.S.M., Lotfibakhshaiesh N., Pour S., Anvari-Yazdi A.F., Abed S.M. How to PrepareBiological Samples and Live Tissues for Scanning Electron Microscopy (SEM). Galen Medical Journal. 2014. 3(2). 63-80.
23. Lundgren E., Roos G. Cell Surface Changes in HeLa Cells as an Indication of Cell Cycle Events. Cancer Research. 1976. 36. 4044-4051.
24. Porter K.R., Fonte V., Weiss G. A Scanning Microscope Study of the Topography of HeLa Cells. Cancer Research. 1974. 34(6). 1385-1394.

/11.jpg)
/12.jpg)
/13.jpg)