Журнал «Здоровье ребенка» Том 15, №3, 2020
Вернуться к номеру
Синдром ятагана: подходы к диагностике и ведению
Авторы: I.Yu. Avramenko, N.S. Kosmynina
Danylo Halytsky Lviv National Medical University, Lviv, Ukraine
Рубрики: Педиатрия/Неонатология
Разделы: Справочник специалиста
Версия для печати
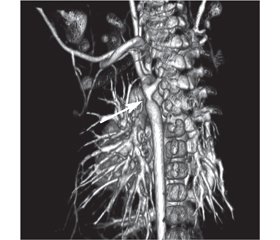
У статті подано клінічний випадок синдрому ятагана в дівчинки віком 3 роки. Захворювання маніфестувало у місячному віці з блідості, перiорального ціанозу та епізоду апное. Дитина була госпіталізована для подальшого обстеження. У дівчинки спостерігався перiоральний ціаноз, SpO2 90 %, частота дихальних рухів 36/хв. Аускультативно в легенях пуерильне дихання, ослаблене справа по передній поверхні грудної клітки. Серцеві тони ритмічні, систолічний шум 2/6 угорі лівого краю грудини і 4/6 у міжлопатковій ділянці зліва. При ехокардіографії діагностовано некритичну коарктацію аорти, дефект міжпередсердної перетинки. При рентгенографії органів грудної клітки в ділянці правого легеневого поля інтенсивна тінь від верхівки до рівня V ребра з нечіткими краями. Тінь серця зміщена вправо. Що в дитини: декстрокардія, декстрапозиція, пневмонія, ателектаз, тимомегалія? При бронхоскопії виявлено обструкцію бронхів верхньої та середньої долі правої легені з густим харкотинням білого кольору. Проведено санацію. Після бронхоскопії нормалізувалася сатурація, але, незважаючи на ліквідацію причини ателектазу, контрольна рентгенографія залишалася без змін. Проведено комп’ютерну томографію органів грудної порожнини з контрастуванням. У дитини діагностовано синдром ятагана в поєднанні з коарктацією аорти і аберантною правою підключичною артерією. У віці 2,5 міс. виконано балонну дилатацію коарктації аорти. У віці 2 років проведено хірургічну корекцію — усунення аномального дренажу легеневих вен та пластику дуги аорти. Інтраопераційна анатомія: мезокардія, праві відділи серця дилатовані, нижня доля правої легені гіпоплазована. Пацієнтка фактично не мала симптомів, характерних для синдрому ятагана, оскільки були відсутні ознаки серцевої недостатності, дівчинка не хворіла на респіраторні захворювання. Діагноз у грудному віці було встановлено завдяки епізоду аспірації, але ателектаз і мезокардія завадили візуалізації характерної рентгенологічної ознаки синдрому ятагана. Тому повне дослідження з використанням комп’ютерної томографії з контрастуванням є обов’язковим для таких пацієнтів.
В статье представлен клинический случай синдрома ятагана у 3-летней девочки. Заболевание манифестировало в месячном возрасте с бледности, периорального цианоза и эпизода апноэ. Ребенок госпитализирован для дальнейшего обследования. У девочки наблюдался периоральный цианоз, SpO2 90 %, частота дыхательных движений 36/мин. Аускультативно в легких пуэрильное дыхание, ослабленное справа по передней поверхности грудной клетки. Сердечные тоны ритмичные, систолический шум 2/6 вверху левого края грудины и 4/6 в межлопаточной области слева. При эхокардиографии диагностированы некритическая коарктация аорты, дефект межпредсердной перегородки. При рентгенографии органов грудной клетки в области правого легочного поля интенсивная тень от верхушки до уровня V ребра с нечеткими краями. Тень сердца смещена вправо. Что у ребенка: декстрокардия, декстрапозиция, пневмония, ателектаз, тимомегалия? При бронхоскопии обнаружена обструкция бронхов верхней и средней доли правого легкого с густой мокротой белого цвета. Проведена санация. После бронхоскопии нормализовалась сатурация, но, несмотря на ликвидацию причины ателектаза, контрольная рентгенография оставалась без изменений. Проведена компьютерная томография органов грудной полости с контрастированием. У ребенка диагностирован синдром ятагана в сочетании с коарктацией аорты и аберрантной правой подключичной артерией. В возрасте 2,5 мес. выполнена баллонная дилатация коарктации аорты. В возрасте 2 лет проведена хирургическая коррекция — устранение аномального дренажа легочных вен и пластика дуги аорты. Интраоперационная анатомия: мезокардия, правые отделы сердца дилатированы, нижняя доля правого легкого гипоплазирована. Наша пациентка фактически не имела симптомов, характерных для синдрома ятагана, поскольку отсутствовали признаки сердечной недостаточности, девочка не болела респираторными заболеваниями. Диагноз в грудном возрасте был установлен благодаря эпизоду аспирации, но ателектаз и мезокардия помешали визуализации характерной рентгенологической картины синдрома ятагана. Поэтому полное исследование с использованием компьютерной томографии с контрастированием является обязательным для таких пациентов.
The article presents a clinical case of scimitar syndrome in a child, who is 3 years old. The manifestation of clinical signs was observed in a child at the age of one month as paleness, perioral cyanosis and episode of apnea. This child was hospitalized for further examination. The girl had perioral cyanosis, SpO2 90 %, respiratory rate is 36/min. Puerile breathing is auscultative in the lungs, and weakened on the right, on the anterior surface of the chest. Heart tones are rhythmic, systolic murmur is 2/6 at the upper left edge of the chest and 4/6 in the interlobular area on the left. Due to echocardiography it was diagnosed non-critical coarctation of the aorta, a secondary defect of the interventricular septum. Chest X-ray: in the area of the right pulmonary field there is an intense shadow from the apex to the level of the 5th rib with fuzzy edges. What does the child have: dextocardia, dextroposition, pneumonia, atelectasis or thymomegaly? Providing the bronchoscopy, it was revealed the obstruction of the bronchi of the upper and middle parts of the right lung with thick murmur of white color. The bronchial tree was rehabilitated. Saturation was normalized after bronchoscopy. But, despite the elimination of the cause of atelectasis, the control radiography was still unchanged. For further diagnosis, CT scan of the chest with contrast was performed. The child was diagnosed with scimitar syndrome in combination with aortic coarctation and aberrant right subclavian artery. Balloon dilation of aortic coagulation was performed at the age of 2.5 months. At the age of 2 years, surgical correction was performed — elimination of abnormal drainage of the pulmonary veins and the aortic arch plastic. Intraoperative anatomy: mesocardia, right heart sections are dilated, lower part of the right lung is hypoplasic. Our patient did not actually have symptoms characteristic of the scimitar syndrome because there were no signs of heart failure, the girl did not have respiratory disease. A diagnosis for a baby at infancy was made due to an aspiration episode. But atelectasis and mesocardia prevented visualization of the characteristic radiological feature of scimitar syndrome. Therefore, a complete study using contrast-enhanced CT is mandatory for such patients.
синдром ятагана; діти; діагностика
синдром ятагана; дети; диагностика
scimitar syndrome; children; diagnosis
- Golubeva M.V., Ilyina N.A., Kagan A.V. Features of pulmonary circulation in children with congenital pulmonary venolobar syndrome. Regional blood circulation and microcirculation. 2019. 18(1). 55-65. https://doi.org/10.24884/1682-6655-2019-18-1-55-65.
- Icek S., Arslan H., Ugurlucan M., Yildiz Y. Scimitar Syndrome: The Curved Turkish Sabre. Seminars in Thoracic and Cardiovascular Surgery: Pediatric Cardiac Surgery Annual. 2014. 7(1). 56-61. doi: 10.1053/j.pcsu.2014.01.003.
- Gustainyte V., Miller M., Towbin T., Towbin A., Neville Kucera J. Scimitar syndrome. Appl. Radiol. 2019. 48(5). 37-39.
- Shamrani A., Sadi R., Ihoury M., Harbi A. Infantile scimitar syndrome with unusual associations. Saudi Medical Journal. 2017. 38(7). 764-767. doi: 10.15537/smj.2017.7.18365. PMID: 28674724. PMCID: PMC5556286.
- Lark M., Cai A., Rideout P., Gregg D., Suranyi P. Novel Technique of Surgical Management of Scimitar Syndrome. Case Reports in Cardiology. 2019. 5. https://doi.org/10.1155/2019/6932680.
- Di Filippo S. Epidemiology and Physiopathology of Scimitar Syndrome. In: The Complete Reference for Scimitar Syndrome. Anatomy, Epidemiology, Diagnosis and Treatment. 2017. 6. 57-66. doi: 10.1016/B978-0-12-810406-4.00005-9.
- Jackson N., Nokes B.T., Sakata K., Cummings K., Vaszar L. An unusual variant of scimitar syndrome predisposing to recurrent pneumonia. Respiratory Medicine Case Reports. 2019. 23(26). 240-243. https://doi.org/10.1016/j.rmcr.2019.01.010.
- Lugones I., Garcia R. A new surgical approach to scimitar syndrome. Ann. Thorac. Surg. 2014. 97. 353-355. doi: 10.1016/j.athoracsur.2013.05.105.
- Brink J., Yong M.S., D’Udekem Y., Weintraub R.G., Brizard C.P., Konstantinov I.E. Surgery for scimitar syndrome: the Melbourne experience. Interact. Cardiovasc. Thorac. Surg. 2015. 20. 31-34. doi: 10.1093/icvts/ivu319. Epub 2014 Oct 6.
- Vida V.L., Padalino M.A., Boccuzzo G., Tarja E., Berggren H., Carrel T., Cicek S. et al. Scimitar syndrome: a European Congenital Heart Surgeons Association (ECHSA) multicentric study. Circulation. 2010. 122. 1159-1166. doi: 10.1161/CIRCULATIONAHA.109.926204. Epub 2010 Sep 7.

/49.jpg)