Статья опубликована на с. 59-76
3G.1. Introduction
In children, UTIs are a frequent health problem, with the incidence only a little lower than that of upper respiratory and digestive infections. Incidence varies depending on age and sex. In the first year of life, mostly the first 3 months, UTI is more common in boys (3.7 %) than in girls (2 %), after which the incidence changes to 3 % in girls and 1.1 % in boys. Paediatric UTI is the most common cause of fever of unknown origin in boys aged < 3 years. The clinical presentation of UTI in infants and young children can vary from fever to gastrointestinal and lower or upper urinary tract symptoms.
Investigation should be undertaken after two episodes of UTI in girls and one in boys (GR: B). The objective is to rule out the unusual occurrence of obstruction, vesicoureteric reflux (VUR) and dysfunctional voiding, e.g. as caused by a neuropathic disorder.
Chronic pyelonephritic renal scarring develops very early in life due to the combination of a UTI, intrarenal reflux and VUR. It sometimes arises in utero due to dysplasia. Although rare, renal scarring may lead to severe long-term complications such as hypertension and chronic renal failure.
VUR is treated with long-term prophylactic antibiotics (GR: B). Surgical re-implantation or endoscopic treatment is reserved for the small number of children with breakthrough infection (GR: B).
For treatment of UTI in children, short courses are not advised and therefore treatment is continued for 5–7 days and longer (GR: A). If the child is severely ill with vomiting and dehydration, hospital admission is required and parenteral antibiotics are given initially (GR: A). For further information please refer to the EAU Paediatric Urology Guidelines.
3G.2. Epidemiology, aetiology and pathophysiology
The urinary tract is a common source of infection in children and infants. It represents the most common bacterial infection in children < 2 years of age [162] (LE: 2a). The outcome of a UTI is usually benign, but in early infancy, it can progress to renal scarring, especially when associated with congenital anomalies of the urinary tract. Delayed sequelae related to renal scarring include hypertension, proteinuria, renal damage and even chronic renal failure, which requires dialysis treatment in a significant number of adults [163] (LE: 2a).
The risk of UTI during the first decade of life is 1 % in males and 3 % in females [6]. It has been suggested that 5 % of schoolgirls and up to 0.5 % of schoolboys undergo at least one episode of UTI during their school life. The incidence is different for children < 3 months of age, when it is more common in boys. The incidence of ABU is 0.7–3.4 % in neonates, 0.7–1.3 % in infants < 3 months of age, and 0.2–0.8 % in preschool boys and girls [6]. The incidence of symptomatic bacteriuria is 0.14 % in neonates, with a further increase to 0.7 % in boys and 2.8 % in girls aged < 6 months. The overall recurrence rate for the neonatal period has been reported to be 25 % [6, 164].
The common pathogenic sources are Gram-negative, mainly enteric, bacteria. Of these, E.coli is responsible for 90 % of UTI episodes [165]. Gram-positive bacteria (particularly enterococci and staphylococci) represent 5–7 % of cases. Hospital-acquired infections show a wider pattern of aggressive bacteria, such as Klebsiella, Serratia and Pseudomonas sp. Groups A and B streptococci are relatively common in new-born infants [166]. There is an increasing trend towards the isolation of S.saprophyticus in UTIs in children, although the role of this bacterium is still debatable [167].
The urinary tract is a sterile space with an impermeable lining. Retrograde ascent is the most common mechanism of infection. Nosocomial infection and involvement as part of a systemic infection are less common [168].
Obstruction and dysfunction are among the most common causes of urinary infection. Phimosis predisposes to UTI [169, 170] (LE: 2a). Enterobacteria derived from intestinal flora colonise the preputial sac, glandular surface and the distal urethra. Among these bacteria are strains of E.coli that express P fimbriae, which adhere to the inner layer of the preputial skin and to uroepithelial cells [171].
A wide variety of congenital urinary tract abnormalities can cause UTIs through obstruction, e.g. urethral valves, ureteropelvic junction obstruction or non-obstructive urinary stasis (e.g. prune belly syndrome, or VUR). More mundane but significant causes of UTIs include labial adhesion and chronic constipation [167].
Dysfunctional voiding in an otherwise normal child may result in infrequent bladder emptying aided by delaying manoeuvres, e.g. crossing legs, sitting on heels [172]. Neuropathic bladder dysfunction (e.g. spina bifida, or sphincter dyssynergia) may lead to post-void residual urine and secondary VUR [164].
The link between renal damage and UTIs is controversial. The mechanism in obstructive nephropathy is self-evident, but more subtle changes occur when there is VUR. Almost certainly, the necessary components include VUR, intrarenal reflux and UTI. These must all work together in early childhood when the growing kidney is likely to be susceptible to parenchymal infection. Later on in childhood, the presence of bacteriuria seems irrelevant to the progression of existing scars or the very unusual formation of new scars. Another confounding factor is that many so-called scars are dysplastic renal tissue which develop in utero [173].
Symptoms are non-specific, and vary with the age of the child and the severity of the disease. Epididymoorchitis is extremely unusual. With scrotal pain and inflammation, testicular torsion has to be considered.
A UTI in neonates may be non-specific and with no localisation. In small children, a UTI may present with gastrointestinal signs, such as vomiting and diarrhoea. In the first weeks of life, 13.6 % of patients with fever have a UTI [174]. Rarely, septic shock is the presentation. Signs of UTI may be vague in small children, but later on, when they are older than 2 years, frequent voiding, dysuria and suprapubic, abdominal or lumbar pain may appear with or without fever.
3G.3. Classification systems
UTIs may be classified as a first episode or recurrent, or according to severity (simple or severe). Recurrent UTI may be subclassified into three groups [168]:
— Unresolved infection: subtherapeutic level of antimicrobial, non-compliance with treatment, malabsorption, resistant pathogens.
— Bacterial persistence: may be due to a nidus for persistent infection in the urinary tract. Surgical correction or medical treatment for urinary dysfunction may be needed.
— Reinfection: each episode is a new infection acquired from periurethral, perineal or rectal flora.
From the clinical point of view, severe and simple forms of UTIs should be differentiated because to some extent the severity of symptoms dictates the degree of urgency with which investigation and treatment are to be undertaken (Table 10).
Severe UTI: severe UTI is related to the presence of fever of > 39 °C, the feeling of being ill, persistent vomiting, and moderate or severe dehydration.
Simple UTI: a child with a simple UTI may have only mild pyrexia, but is able to take fluids and oral medication. The child is only slightly or not dehydrated and has a good expected level of compliance. When a low level of compliance is expected, such a child should be managed as one with a severe UTI.
3G.4. Diagnostic evaluation
3G.4.1. Physical examination
It is mandatory to look for phimosis, labial adhesion, signs of pyelonephritis, epididymo-orchitis, and stigmata of spina bifida, e.g. hairy patch on the sacral skin. The absence of fever does not exclude the presence of an infective process.
3G.4.2. Laboratory tests
The definitive diagnosis of infection in children requires a positive urine culture [168, 175]. Urine must be obtained under bacteriologically reliable conditions when undertaking a urine specimen culture [176]. A positive urine culture is defined as the presence of > 100,000 cfu/mL of one pathogen. The urine specimen may be difficult to obtain in a child < 4 years old, and different methods are advised because there is a high risk of contamination [177, 178].
3G.4.2.1. Collection of the urine
Suprapubic bladder aspiration: this is the most sensitive method, even though urine may be obtained in 23–99 % of cases [168, 177].
Bladder catheterisation: this is also a very sensitive method, even though there is the risk of introduction of nosocomial pathogens [168, 179].
Plastic bag attached to the genitalia: prospective studies have shown a high incidence of false-positive results, ranging from 85–99 % [168, 177]. It is helpful when the culture is negative [168, 177] and has a PPV of 15 % [176]. To obtain a urine sample in the best condition in children < 2 years of age (girls and uncircumcised boys without sphincteric control), it is better to use suprapubic bladder aspiration or bladder catheterisation. In older children with sphincteric control, MSU collection is possible and reliable [177].
3G.4.2.2. Quantification of bacteriuria
The final concentration of bacteria in urine is directly related to the method of collection, diuresis, and method of storage and transport of the specimen [175]. The classical definition of significant bacteriuria of > 105 cfu/mL is still used and depends on the clinical environment [175, 178].
The presence of pyuria (> 5 leukocytes per field) and bacteriuria in a fresh urine sample reinforce the clinical diagnosis of UTI [178].
In boys, when the urine is obtained by bladder catheterisation, the urine culture is considered positive with > 104 cfu/mL. Even though Hoberman [180] has identified a microorganism in 65 % of cases with colony counts between 10,000 and 50,000 cfu/mL,
there was a mixed growth pattern suggesting contamination. In these cases, it is better to repeat the culture or to evaluate the presence of other signs, such as pyuria, nitrites or other biochemical markers [175]. The collection of MSU or in a collecting bag of > 105 cfu/mL
is considered positive [176] (Table 11).
3G.4.2.3. Other biochemical markers
The presence of other biochemical markers in a urine sample are useful to establish the diagnosis of UTI [168]. The most frequent markers are nitrite and leukocyte esterase usually combined in a dipstick test.
Nitrite: This is the degradation product of nitrate in bacterial metabolism, particularly in Gram-negative bacteria. When an infection is caused by Gram-positive bacteria, the test may be negative [168, 176]. Limitations of the nitrite test include:
— not all uropathogens reduce nitrate to nitrite, e.g. P.aeruginosa, or enterococci;
— even nitrite-producing pathogens may show a negative test result, due to the short transit time in the bladder in cases of high diuresis and urine dilution, e.g. neonates;
— the nitrite test has a sensitivity of only 45–60 %, but a very good specificity of 85–98 % [168, 178, 181].
Leukocyte esterase: This is produced by the activity of leukocytes. The test for leukocyte esterase has a sensitivity of 48–86 % and a specificity of 17–93 % [168, 178, 180, 181].
A combination of nitrite and leukocyte esterase testing improves sensitivity and specificity, but carries the risk of false-positive results [181].
The dipstick test has become useful to exclude rapidly and reliably the presence of a UTI, provided both nitrite and leukocyte esterase tests are negative. If the tests are positive, it is better to confirm the results in combination with the clinical symptoms and other tests [178, 181].
Bacteriuria without pyuria may be found:
— in bacterial contamination;
— in colonisation (ABU);
— when collecting a specimen before the onset of an inflammatory reaction.
In such cases, it is advisable to repeat the urinalysis after 24 h to clarify the situation. Even in febrile children with a positive urine culture, the absence of pyuria may cast doubt on the diagnosis of UTI. Instead, ABU with a concomitant septic focus responsible for the febrile syndrome has to be considered.
Bacteriuria without pyuria is found in 0.5 % of specimens. This figure corresponds well with the estimated rate of ABU in childhood [180, 182] (LE: 2a).
Pyuria without bacteriuria may be due to:
— incomplete antimicrobial treatment of UTI;
— urolithiasis and foreign bodies;
— infections caused by M. tuberculosis and other fastidious bacteria, e.g. C.trachomatis.
Thus, either bacteriuria or pyuria may not be considered reliable parameters to diagnose or exclude UTI. Their assessment can be influenced by other factors, such as the degree of hydration, method of specimen collection, mode of centrifugation, volume in which sediment is resuspended and subjective interpretation of results [183]. However, according to Landau et al. [184], pyuria in febrile children is indicative of acute pyelonephritis.
For all of these reasons, in neonates and children < 6 months of age, either pyuria, bacteriuria or the nitrite test, separately, have minimal predictive value for UTI [185, 186] (LE: 3). In contrast, the PPV of significant Gram staining with pyuria is 85 % [180] (LE: 2b). In older children, pyuria with a positive nitrite test is more reliable for the diagnosis of UTI, with a PPV of 98 %.
Combining bacteriuria and pyuria in febrile children, the findings of > 10 WBC/mm3 and > 50,000 cfu/mL in a specimen collected by catheterisation are significant for a UTI, and discriminate between infection and contamination [180, 185].
C-reactive protein: Although non-specific in febrile children with bacteriuria, C-reactive protein seems to be useful in distinguishing between acute pyelonephritis and other causes of bacteriuria. It is considered significant at a concentration > 20 μg/mL.
Urinary N-acetyl-b-glucosaminidase: This is a marker of tubular damage. It is increased in febrile UTI and may become a reliable diagnostic marker for UTIs, although it is also elevated in VUR [187].
IL-6: The clinical use of urinary concentrations of IL-6 in UTIs [188] is still at the research stage.
3G.4.3. Imaging of the urinary tract
A gold standard imaging technique has to be cost-effective, painless, safe, and have minimal or no radiation, as well as have the ability to detect any significant structural anomaly. Current techniques do not fulfil all such requirements.
3G.4.3.1. Ultrasound
Ultrasound (US) has become very useful in children because of its safety, speed and high accuracy in identifying the anatomy and size of the renal parenchyma and collecting system [189]. It is subjective and therefore operator-dependent, and gives no information on renal function. However, scars can be identified, although not as well as with Tc-99m DMSA scanning [189, 190] (LE: 2a). This technique has been shown to be very sensitive and excretory urography must be reserved only for when images need to be morphologically clarified [191] (LE: 2a).
3G.4.3.2. Radionuclide studies
Tc-99m DMSA is a radiopharmaceutical that is bound to the basement membrane of proximal renal tubular cells; half of the dose remains in the renal cortex after 6 h. This technique is helpful in determining functional renal mass and ensures an accurate diagnosis of cortical scarring by showing areas of hypoactivity, which indicates lack of function. A UTI interferes with the uptake of this radiotracer by the proximal renal tubular cells, and may show areas of focal defect in the renal parenchyma. A star-shaped defect in the renal parenchyma may indicate an acute episode of pyelonephritis. A focal defect in the renal cortex usually indicates a chronic lesion or a renal scar [192–194] (LE: 2a).
Focal scarring or a smooth uniform loss of renal substance as demonstrated by Tc-99m DMSA is generally regarded as being associated with VUR (reflux nephropathy) [195, 196]. However, Rushton et al. [197] have stated that significant renal scarring may develop, regardless of the existence or absence of VUR. Ransley and Risdon [198] have reported that Tc-99m DMSA shows a specificity of 100 % and sensitivity of 80 % for renal scarring.
The use of Tc-99m DMSA scanning can be helpful in the early diagnosis of acute pyelonephritis. About 50–85 % of children show positive findings in the first week. Minimal parenchymal defects, when characterised by a slight area of hypoactivity, can resolve with antimicrobial therapy [199, 200]. However, defects lasting > 5 months are considered to be renal scarring [201] (LE: 2a).
Tc-99m DMSA scans are considered more sensitive than excretory urography and US in the detection of renal scars [202–205]. It remains questionable whether radionuclide scans can substitute echography as a first-line diagnostic approach in children with a UTI [206, 207].
3G.4.3.3. Cystourethrography
Conventional voiding cystourethrography (VCU): This is the most widely used radiological exploration for the study of the LUT and especially of VUR. It is considered mandatory in the evaluation of UTIs in children < 1 year of age. Its main drawbacks are the risk of infection, the need for retrogrades filling of the bladder, and the possible deleterious effect of radiation on children [208]. In recent years, tailored low-dose fluoroscopic VCU has been used for the evaluation of VUR in girls to minimise radiological exposure [209]. VCU is mandatory in the assessment of febrile childhood UTI, even in the presence of normal US. Up to 23 % of these patients may reveal VUR [210].
Radionuclide cystography (indirect): This investigation is performed by prolonging the period of scanning after the injection of Tc-99m diethylene triamine pentaacetate (DTPA) or mercaptoacetyltriglycine (MAG-3) as part of dynamic renography. It represents an attractive alternative to conventional cystography, especially when following patients with reflux, because of its lower dose of radiation. Disadvantages are poor image resolution and difficulty in detecting LUT abnormalities [211, 212].
Cystosonography: Contrast-material-enhanced voiding US has been introduced for the diagnoses of VUR without irradiation [207, 212]. Further studies are necessary to determine the role of this new imaging modality in UTI.
3G.4.3.4. Additional imaging
Excretory urography remains a valuable tool in the evaluation of the urinary tract in children, but its use in UTIs is debatable unless preliminary imaging has demonstrated abnormalities that require further investigation. The major disadvantages in infants are the risks of side-effects from exposure to contrast media and radiation [213]. However, the role of excretory urography is declining with the increasing technical superiority of CT [214] and MRI. However, the indications for their use is still limited in UTI.
3G.4.3.5. Urodynamic evaluation
When voiding dysfunction is suspected, e.g. incontinence, residual urine, increased bladder wall thickness, urodynamic evaluation with uroflowmetry, (video) cystometry, including pressure flow studies, and electromyography should be considered.
3G.4.4. Schedule of investigation
Screening of infants for ABU is unlikely to prevent pyelonephritic scar formation, as these usually develop very early in infancy. Only a minority of children with a UTI have an underlying urological disorder, but when present, such a disorder can cause considerable morbidity. Thus, after a maximum of two UTI episodes in a girl and one in a boy, investigations should be undertaken (Figure 4), but not in the case of ABU [210–213, 215, 216]. The need for DTPA/MAG-3 scanning is determined by the US findings, particularly if there is suspicion of an obstructive lesion.
3G.5. Disease management
Treatment has four main goals:
— elimination of symptoms and eradication of bacteriuria in the acute episode;
— prevention of renal scarring;
— prevention of a recurrent UTI;
— correction of associated urological lesions.
3G.5.1. Severe UTIs
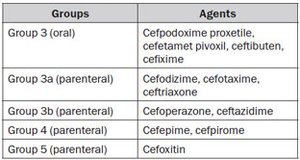
A severe UTI requires adequate parenteral fluid replacement and appropriate antimicrobial treatment, preferably with cephalosporins (third generation). If a Gram-positive UTI is suspected by Gram stain, it is useful to administer aminoglycosides in combination with ampicillin or amoxycillin/clavulanate [217] (LE: 2a). Antimicrobial treatment has to be initiated on an empirical basis, but should be adjusted according to culture results as soon as possible. In patients with an allergy to cephalosporins, aztreonam or gentamicin may be used. When aminoglycosides are necessary, serum levels should be monitored for dose adjustment.
Chloramphenicol, sulphonamides, tetracyclines, rifampicin, amphotericin B and quinolones should be avoided. The use of ceftriaxone must also be avoided due to its undesired side effect of jaundice.
A wide variety of antimicrobials can be used in older children, with the exception of tetracyclines (because of tooth staining). Fluorinated quinolones may produce cartilage toxicity [218], but if necessary, may be used as second-line therapy in the treatment of serious infections, because musculoskeletal adverse events are of moderate intensity and transient [219, 220]. For a safety period of 24–36 h, parenteral therapy should be administered. When the child becomes afebrile and is able to take fluids, he/she may be given an oral agent to complete the 10–14 days of treatment, which may be continued on an outpatient basis. This provides some advantages, such as less psychological impact on the child and more comfort for the whole family.
It is also less expensive, well tolerated and eventually prevents opportunistic infections [180].
The preferred oral antimicrobials are: trimethoprim (TMP), co-trimoxazole (TMP plus sulphamethoxazole), an oral cephalosporin, or amoxycillin/clavulanate. However, the indications for TMP are declining in areas with increasing resistance.
In children < 3 years of age, who have difficulty taking oral medications, parenteral treatment for 7–10 days seems advisable, with similar results to those with oral treatment [221].
If there are significant abnormalities in the urinary tract (e.g. VUR, or obstruction), appropriate urological intervention should be considered. If renal scarring is detected, the patient will need careful follow-up by a paediatrician in anticipation of sequelae such as hypertension, renal function impairment, and recurrent UTI.
An overview of the treatment of febrile UTIs in children is given in Figure 5 and the dosing of antimicrobial agents is outlined in Table 12 [222].
3G.5.2. Simple UTIs
A simple UTI is considered to be a low-risk infection in children. Oral empirical treatment with TMP, an oral cephalosporin or amoxycillin/clavulanate is recommended, according to the local resistance pattern. The duration of treatment in uncomplicated UTIs treated orally should be 5–7 days [223, 224] (LE: 1b). A single parenteral dose may be used in cases of doubtful compliance and with a normal urinary tract [225] (LE: 2a). If the response is poor or complications develop, the child must be admitted to hospital for parenteral treatment [226].
3G.5.3. Prophylaxis
If there is an increased risk of pyelonephritis, e.g. VUR, and recurrent UTI, low-dose ABP is recommended [227, 228] (LE: 2a). It may also be used after an acute episode of UTI until the diagnostic work-up is completed. The most effective antimicrobial agents are: nitrofurantoin, TMP, cephalexin and cefaclor [227].
Acknowledgement
With our grateful thanks, the chapter on UTIs in children was updated also by Jorge Caffaratti Sfulcini, Paediatric Urology, Fundació Puigvert, Barcelona, Spain, as co-author.
/61-2.jpg)
/63-2.jpg)
/66-2.jpg)

/68-2.jpg)
/69.jpg)
/71.jpg)
/74-2.jpg)
/75.jpg)
/76-1.jpg)
/76-3.jpg)