Журнал «Боль. Суставы. Позвоночник» 1 (01) 2011
Вернуться к номеру
The Role of Bone Biopsy in Bone Diseases — Analytical Methods — A Clinical Evaluation of 152 Consecutive Cases over a Period of 12 Years
Авторы: Resch H., Trubrich A., Bittighofer Ch., Kocijan R., Pirker Th., Patsch J., Muschitz Ch.
Medical University, Vienna, Department II Rheumatology/Osteology & Gastroenterology
KH Barmherzige Schwestern (St. Vincent Hospital), Academic Teaching Hospital of the Medical University Vienna, Austria
Рубрики: Семейная медицина/Терапия, Ревматология, Травматология и ортопедия, Неврология
Версия для печати
 Introduction and background
Introduction and background
Osteoporosis has been characterised and defined as a systemic metabolic bone disease causing a deterioration of skeletal tissue and mechanical competences [1–3]. The advanced understanding of osteoporosis, however, has changed markedly during the last years. The impact of the classic histopathology has been replaced by a structural, mechanical, genetic and material related understanding. From the clinical standpoint, non-invasive diagnostic procedures have become established and seem to make it very easy to get a correct clinical diagnosis [4–7].
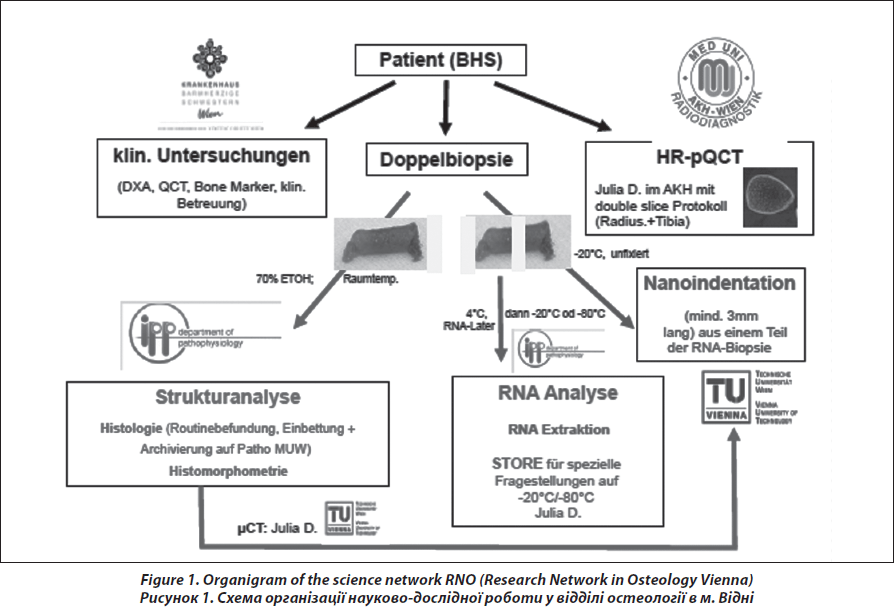
Bone biopsy has been restricted so far to untypical, unclear and complicated cases in evidence-based guidelines on diagnosis and treatment of osteoporosis [8, 9]. However, with respect to the tremendous progress in structural and genetical analysis of bone tissue and on the other hand with respect to the progress in the development of new pharmacotherapy, the indication for the assessment of bone tissue has switched to younger patients, even children, males and patients with a substantial discrepancy in the clinical exploration. There is a permanent controversial discussion about performing bone biopsies, the way of preparing and conserving the sample and their correct interpretation to differentiate between low-turnover and high-turnover osteoporosis and different architectural patterns in the cortical and trabecular area with impact on therapeutic decisions [10–14]. In this context, our report gives a description of the science network, the clinical procedure and the impact of the role and relevance of bone biopsy in a medical center, which is responsible for different clinical tasks; basic and clinical research, students training and clinical routine in the management of skeletal diseases (Fig. 1). Subject of the manuscript is a descriptive analysis on frequency and indications for bone biopsy, quality of the specimens, histopathological diagnosis, and the possibilities of analysis on the different bone levels depicting consequences for therapeutic strategy for the patients.
Patients
In the time period between April 1995 and December 2010, a total of 152 transiliac bone biopsies were performed at the II. Medical Department of Rheumatology/Osteology of the St. Vincent Hospital Vienna in cooperation with the Medical University of Vienna. The indications for a bone biopsy were as following:
Differential diagnosis of bone diseases
— In particular Juvenile (premenopausal) Osteoporosis (DD Osteogenesis imperfecta).
— Male osteoporosis and fractures after inadequate traumas.
— Clinical discrepancies — fractures at normal or osteopenic BMD measurements.
— Hyperostosis.
— Fragility fractures in young patients.
— Non responders to antiresorptive therapy.
— Scientific investigations & studies.
The aims of the procedure in general are:
— Analysis of microarchitecture and material properties (mineralization).
— Analysis of mRNA extraction on tissue level.
— Gender-specific differences in structure and metabolic dynamics.
— Initiation of specific therapies.
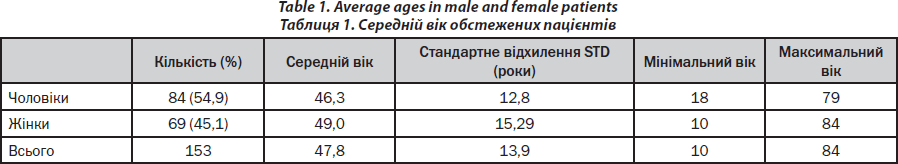
A brief characterisation of the patients is given in Table 1. In the observation period, about 80–100 consultations of patients occurred weekly (Monday — Friday) for bone diseases in the outpatient and inpatient wards. The majority of bone biopsies (90 %) was performed by one single investigator (PHK). Transiliac bone biopsy was performed as described in one of our publications [15] with respect to the technique of Bordier et al. [16].
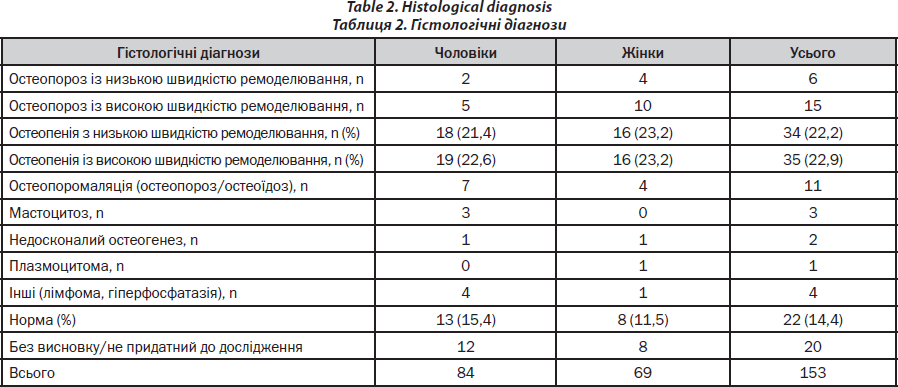
The spectrum of diagnoses is represented in Table 2.
Indications and Clinical Evaluation
Following the study approval by the local ethics committee, patients with bone diseases are recruited at the outpatient clinic of the II Medical Department, St. Vincent Hospital Vienna, Austria.
All patients have to give written informed consent prior to any study or routine-related procedures.
Medical history details including the previous use of osteotropic drugs, concomitant medication, life-style habits (e.g. smoking), fractures including trauma history and parental fractures are recorded in detail and implemented by the FRAX calculation tool. Inclusion criteria are adapted from major interventional trials in female and male osteoporosis [17, 18]. Exclusion criteria for the biopsy procedure include the previous use of any specific antiosteoporotic substances over a longer time period (more than 12 months) other than vitamin D and calcium supplements (e.g. bisphosphonates). Moreover, hypogonadism, thyroid disorders, the use of corticosteroids, thyroid hormones, antiepileptic substances or any malignoma history within the last five years exclude patients from enrolment of the procedure.
Clinical Examination, Densitometry and X-Ray-Imaging
Prior to each bone biopsy physical examination is performed, body weight and patient height are assessed using a stadiometer with integrated weighing scale (BWB 700, Tanita). Fasting blood samples are drawn from all patients between 8:00 and 10:00 a.m. Routine blood and urine analyses are performed at the Vienna-based Central Laboratory of the St. Vincent Group. Routine blood tests include a whole blood count, serum potassium, sodium, calcium and phosphate as well as PTH, 25-(OH)-vitamin D, TSH, kidney and liver function parameters and total testosterone measurements in male patients. Calcium and phosphate excretion is measured from a 24-hour-urine collection. Moreover patients undergo dual-X-ray-absorptiometry (DXA) scanning of the hip and spine (Lunar iDXA, GEHealthcare). In case of inconclusive DXA measurements due to degenerative alterations QCT of lumbar spine and hip is performed. To complete clinical work-up, vertebral fracture status is analyzed by anterior-posterior and lateral x-ray-studies of the thoracic and lumbar spine. The images are read by an experienced radiologist trained for Genant scoring [19].
Biochemical Bone Markers
Per patient 2 ml serum is stored in 1 ml voids at –70°C for batched bone marker double analysis at the Department of Laboratory Medicine of the Medical University of Vienna. Bone markers are measured using electrochemiluminescence-immunoassays (ECLIA; beta-CrossLaps (CTX), N-MID Osteocalcin (OCN), total N-terminal type I procollagen propeptide (P1NP); all Roche Diagnostics) on a Modular Analytics E170 device (Roche Diagnostics).
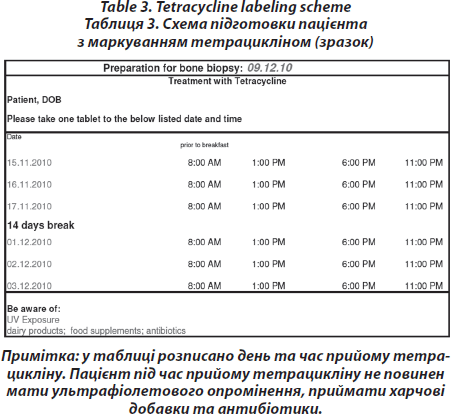
Pre-interventional procedure — Labelling with tetracycline
On days –21/–20/–19 and days –8/–7/–6 before biopsy, 2000 mg tetracycline are administered orally to visualise mineralisation. (Tetracycline double labelling is performed routinely to retain the option for a later post hoc quantitative analysis of the bone specimen and dynamic histomorphometry) [16]. However, this procedure is not necessary to gete a histopathological diagnosis, which can also be achieved by static histomorphometry, without labelling. Mineralized bone histology after tetracycline double labelling is the only way to establish absence or presence of mineralization defect or evident osteomalacia with or without concomitant osteopenia (Tab. 3).
Technique of transiliac Bone Biopsy
Prior to the biopsy the patient is monitored (blood pressure, oxygen saturation) and receives oxygen continuously. Sedoanalgesia is performed by using midazolame and propofol (1%) intravenously and lidocaine (1%) subcoutaneously.
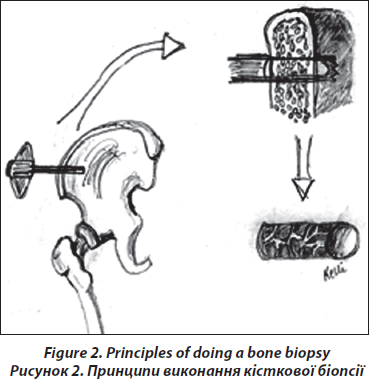
The transiliac biopsy is taken after incision of the skin from an area situated 5-8 cm below the anteriorsuperior iliac spine and 8 cm below the summit of the iliac crest using a manual trocar with 6-mm inner diameter. In order to perform histomorphometry and structural analysis as well as gene expression testing, each patient undegoes two parallel-oriented, left transiliac bone biopsies (Fig. 2). Using a trephine needle, all biopsies are carried out under sterile conditions at the local operating theatre. Both biopsy cylinders (inner diameter = 7mm) are first visually examined. This method produces a sample with cortex at both ends (6 : 8 mm) which is a representative volume of iliac bone in its entirety, i.e. as an organ, and permits the measurement of trabecular and cortical parameters and the transition areas like the endoresoptive area (Fig. 3). The procedure lasts about 12–15 minutes.
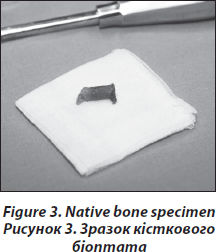 The larger, more intact sample is selected for subsequent histomorphometric and structural analysis and is placed in 70% ethanol. The second biopsy is immediately submerged in RNA-Later (Ambion) after dissection of cutaneous, fatty or muscular fragments, stored as instructed by the manufacturer and shipped on dry ice to the Medical University of Vienna.
The larger, more intact sample is selected for subsequent histomorphometric and structural analysis and is placed in 70% ethanol. The second biopsy is immediately submerged in RNA-Later (Ambion) after dissection of cutaneous, fatty or muscular fragments, stored as instructed by the manufacturer and shipped on dry ice to the Medical University of Vienna.
After biopsy, patients are advised to maintain bed rest with the biopsy site on a sand sack for a period of 6 h and patients should refrain from hard work for at least 3–4 days. Normally, the 3–4 stitching are removed after 14 days. Local side effects are seldom, mostly due to early movements of the patients including local hematoma. None of the patients had a post interventional wound infection so far.
Histology and Histomorphometry
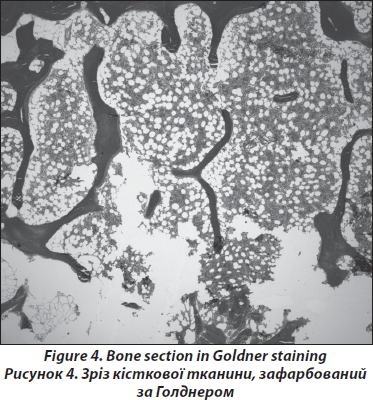
Processing of the bone samples is performed without decalcification as described previously [19, 20]. Sections of 5–8m thickness are cut, and Goldner and Giemsa staining are performed; Specimen processing for histology is performed according to Roschger et al. [22, 23]. All ‘structure’-biopsies are ethanol-fixed, dehydrated and polymethylmethacrylate-(PMMA)-embedded. The sections are prepared with 2-methoxyethyl-acetate before being stained with a modified Goldner’s Trichrome method. Histological analyses are performed according to Parfitt et al. [24] on the whole area of the bone sections. In order to exclude malignant causes of osteoporosis such as lymphoma or mastocytosis, a certified pathologist performs routine assays (Fig. 4). A light microscope (Axiophot, Zeiss, Oberkochen, Germany) equipped with a Zeiss AxioCam videocamera is used to obtain digital images of the sections. The images are analyzed using standard procedures (NIH Image software versions 1.62 and 1.63, Wayne Rasband, National Institutes of Health, Bethesda, MD) on a Power Macintosh G4. For the male patients structural parameters and static parameters of bone formation and resorption are usually compared with published normative data from men aged between 41 and 50 years [25].
Analytical histomorphometry is performed by BIOQUANT Image Analysis Corporation (Nashville,TN) using the BIOQUANT OSTEO bone biology research system, version 8.40.10. For each section, 25 systematically random fields of view are imaged with a 20X objective from within the trabecular compartment. Histomorphometric data (Fig. 5) are reported according to the standardized nomenclature [24]. In addition, marrow volume (Ma.V) is calculated as tissue volume minus bone volume. Fat volume (Fat V) is calculated as the total adipocyte volume within bone marrow volume.
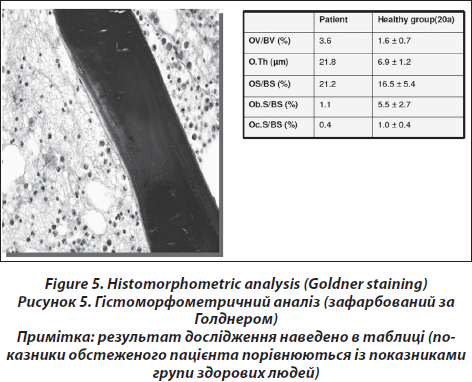
As an alternative semiquantitative approach osteoporomalacia (osteoporosis and osteomalacia) is distinguished from high-turnover osteoporosis by quantifying and characterising osteoblasts. High-turnover osteoporosis is characterised by about 30 % of osteoid surface covered with cubic osteoblasts and high-normal or increased number of osteoclasts. Mineralizing surface or bone surface and activation frequency is increased; whereas mineral apposition rate and mineralization lag time is normal. Low-turnover osteopenia is characterized by few remodeling foci and reduction in osteoblast and osteoclast number. Osteoidosis and fibro-osteoclasia may be considered as indicators for renal osteodystrophy if impaired renal function is known (this information is always provided to the osteopathologist); if this is not the case, this histological finding suggests primary hyperparathyreoidism.
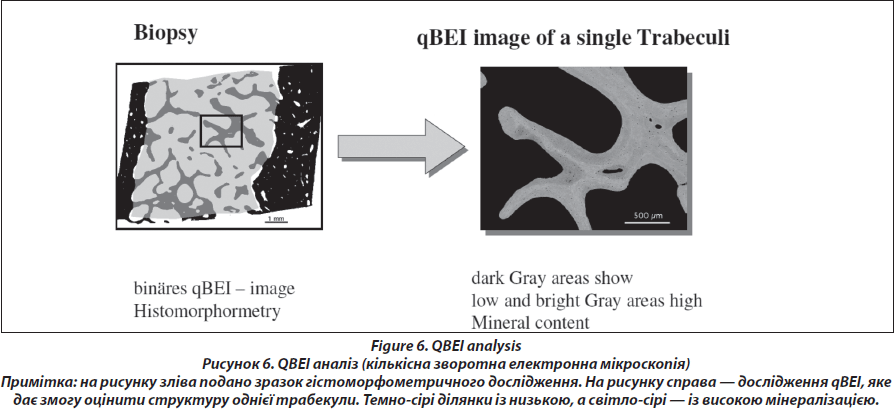
Quantitative backscattered electron imaging (Fig. 6.)
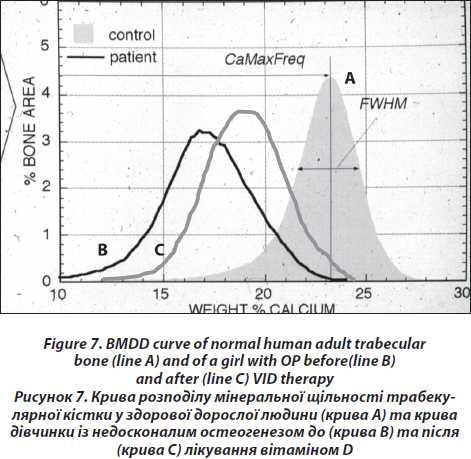
The block samples embedded in polymethylmethacrylate (PMMA) containing cancellous and cortical bone are prepared by grinding and polishing to receive plane and parallel surfaces. Subsequently, the surface plane containing bone tissue is coated by carbon (using vacuum evaporation) for qBEI analysis in the scanning electron microscope. Cancellous (Cn.) and cortical (Ct.) bone mineralization density distribution (BMDD) are determined by qBEI using a digital scanning electron microscope (DSM 962, Zeiss, Oberkochen, Germany) equipped with a four-quadrant semiconductor backscattered electron (BE) detector as described previously [26]. The accelerating voltage of the electron beam is adjusted to 20 kV, the probe current to 110 pA, and the working distance to 15 mm. The entire cancellous and cortical bone areas are imaged at x 50 nominal magnification (corresponding to a pixel resolution of 4 μm/pixel), using a scan speed of 100 sec/frame, resulting in digital calibrated BE images of 512 x 512 pixels. From the digital images, grey level histograms are deduced, displaying the percentage of bone area occupied by pixels of a certain grey level. The transformation of these into calcium wt% histograms leads to a bin width of 0.17wt% calcium. A technical precision of 0.3 % is achieved. The BMDD parameters e.g., the mean (weighted mean) CaMean and the most frequent calcium concentration CaPeak (mode, peak position of the BMDD) in the sample, the width of the distribution CaWidth (full width at half maximum) reflecting the heterogeneity in matrix mineralization, the fraction of low mineralized bone (CaLow), which is the percentage of the area below 17.68 wt% Ca (corresponding to the 5th percentile of reference BMDD) and the fraction of fully mineralized bone (CaHigh), which is the portion of the area above 25.30 wt% Ca (corresponding to the 95th percentile of reference BMDD) [27, 28] are derived from the histogram. The BMDD values obtained from the patients are compared to those of the reference BMDD of normal adults (Fig. 7) [28, 29].
Micro-computed Tomography (μ-CT)
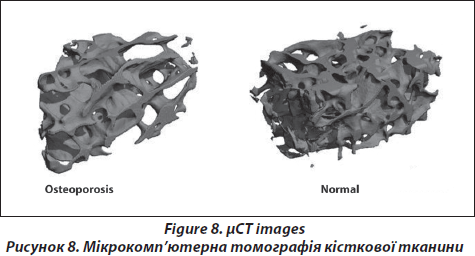
The micro-tomographic imaging system (μCT40, Scanco Medical AG, Brьttisellen, Switzerland) is equipped with a 5μm focal spot X-ray tube as a source. A two-dimensional CCD coupled to a thin scintillator as a detector permitted acquisition of 206 tomographic images in parallel. The long axis of the PMMA-embedded biopsy is oriented along the rotation axis of the scanner. The X-ray tube is operated at 70kVp and 114μA with an integration time set to 200ms. Scans are performed at an isotropic, nominal resolution of 10μm (high resolution mode). The image data are filtered using a Gaussian filter (sigma 1.2, support 1) to partially suppress noise in the volume. The mineralized tissue is segmented from soft tissue by a global threshold procedure with a value set to 22 % of the maximum greyscale value. A special automatic contouring algorithm is used to automatically detect the envelope of the biopsy, followed by a 3D erosion algorithm to define the trabecular bone volume of interest (VOI) within the biopsy and to exclude any cortical bone. Morphometric indices are determined for the nine trabecular bone compartment using a direct 3D approach [30] and included bone volume (BV/TV), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), trabecular number (Tb.N), connectivity density (Conn.D) and the structure model index (SMI).
RNA extraction, cDNA and RealTime PCR
Using RNAse-free instruments, a small cube of trabecular bone (approx. 5 x 5 x 5 mm) is cut out of the middle part of the intact biopsy at a laminar flow bench. To reduce marrow content, the cube is repeatedly rinsed with RNA-Later. Together with two small steel beads, the bone sample are transferred to an RNAse-free Eppendorf tube and are flash-frozen in liquid nitrogen. For tissue homogenization, the cooled tubes are placed in a grinding mill (3 minutes; 30Hz). Subsequently RNA extraction is performed on the basis of a guanidinium thiocyanate-phenol-chloroform protocol (Trizol, Invitrogen) [31]. Apart from double precipitation and centrifugation at the isopropanol stage (12000 g; 10 minutes; 4 °C), the manufacturer‘s protocol is followed. RNA quality and quantity are checked by electrophoresis and photometry at 260 nm and 280 nm. The 260/280nm absorbance ratios ranged from 1.5 to 2. cDNA is synthesized from 4 μg of total RNA using a cDNA synthesis kit (High Capacity cDNA reverse transcription kit, Applied Biosystems). Realtime PCR is performed using assay-on-demand primers and probes following the manufacturer’s instructions. Per reaction the amplification mixture (20 μl) consisted of 9 μl cDNA (dilution 1 : 10), 10 μl mastermix buffer (TaqMan Universal PCR Mastermix) and 1 μl probe i.e. Wnt10b Hs00559664_m1), runx2 (Hs00231692_m1), osterix (Hs00541729_m1), osteocalcin (Hs00609452_g1), SOST (Hs00228830_m1), RANKL (Hs00243519_m1), OPG (Hs00171068_m1) and GAPDH (Hs99999905_m1). Using a thermal cycler (ABI Prism Sequence Detection System 7700, Applied Biosystems) 10 cycler conditions were 50 °C for 2 min and 94 °C for 2 min, followed by 40 cycles with 94 °C for 15 s and 60 °C for 30 s. Amplification curves are visually checked for exponentially and thresholds are set at 0.15 units for RANKL, OPG, OSX and runx2. The threshold for GAPDH is 0.03 units, whereas the threshold for wnt, osteocalcin and SOST is set at 0.07 units. All experiments are performed in triplicates and are normalized to the housekeeping gene GAPDH. GAPDH is chosen as housekeeping gene because of its repeated use in human gene expression studies addressing osteoporosis research questions [32]. The results are calculated applying the µ-CT method and are presented in fold increase relative to GAPDH expression (Fig. 8).
Conclusion
Despite all progress in non-invasive diagnostic procedures for metabolic bone diseases such as osteoporosis, there is still a small subgroup of female and male patients who may benefit from adding bone biopsy into the diagnostic procedure. Most of the patients with osteoporosis are mainly managed using serum biochemical markers or non-invasive imaging techniques. These patients are categorized as untypical cases, i.e. males and pre- and postmenopausal females with fragility fractures, individuals in whom conventional non-invasive diagnostic procedures cannot explain the aetiology of the bone disease, or their «non-responders» status to treatment.
Limitations of bone biopsies can be optimised with frequent training of the biopsy technique and better understanding oft the analytical information that can be obtained from bone biopsies. Since new therapeutic agents have been developed which suppress high osteoclast turnover and osteoclast inductive therapies which stimulate predominantly bone formation. Basically bone biopsies should be performed when pathogenic mechanisms are unclear. Further, changes in bone quality and material properties, such as microarchitecture, matrix quality, and mineral quality as contributing factors to bone fragility — still require invasive analysis of bone tissue and cannot be described by non-invasive techniques.
1. Kruse H.-P., Kuhlencordt F. Grundzьge der Osteologie. — Berlin: Heidelberg; New York: Springer-Verlag; Tokyo,1984.
2. Malluche H.H., Faugere M.-C. Bone biopsies: histology and histomorphometry of bone // Metabolic bone disease and clinically related disorders / Avioli L.V., Krane S.M. (eds). — Philadelphia: WB Saunders Company, 1990. — Р. 283-328.
3. Meunier P.J. Assessment of bone turnover by histomorphometry in osteoporosis // Osteoporosis — etiology, diagnosis and management / Riggs B.L., Melton L.J. (eds). — New York: Raven Press, 1988. — Р. 317-332.
4. Kann P.H. Bone densitometry and ultrasound studies of the bone: methods, indications and efficacy // Orthopдde. — 2001. — 30. — 437-443.
5. Pfeilschifter J., Kann P.H. Evaluation of osteoporosis // Z. Gastroenterol. — 2002. — 40 (Suppl. 1). — S46-S56.
6. Ettinger MP Aging bone and osteoporosis: strategies for preventing fractures in the elderly // Arch. Intern. Med. — 2003. — 163. — 2237-2246.
7. Kenny A.M., Joseph C., Taxel P., Prestwood K.M. Osteoporosis in older men and women // Conn. Med. — 2003. — 67. — 481-486.
8. Scheidt-Nave C., Baum E., Dцren M., Hadji P., Keck E., Minne E., Seibel M. DVO-Leitlinie Osteoporose bei postmenopausalen Frauen // Osteologie. — 2003. — 12. — 13-41.
9. Fassbender W.J., Scheidt-Nave Ch., Pfeilschifter J. Dachverband der Deutschsprachigen Osteologischen Fachgesellschaften Evidence-based clinical practice guidelines for diagnosis and treatment of osteoporosis // Dtsch Med. Wochenschr. — 2003. — 128. — 1615-1616.
10. Hauge E., Mosekilde L.E., Melsen F. Missing observations in bone histomorphometry on osteoporosis: implications and suggestions for an approach // Bone. — 1999. — 25. — 389-395.
11. Ziegler R Osteoporose: aktuelle Diagnostik und Therapie // Orthopдdische Praxis. — 2002. — 38. — 570-577.
12. Mehl B., Delling G., Schlindwein I., Heilmann P., Voia C., Ziegler R., Nawroth P., Kasperk C. Do markers of bone metabolism reflect the presence of a high- or low-turnover state of bone metabolism? // Med. Klin. — 2002. — 97. — 588-594.
13. Allolio B. Role of bone histology in the determination of bone metabolism // Med. Klin. — 2003. — 98. — 110.
14. Barthel H.R., Seibel M.J. Role of bone histology in the determination of bone metabolism // Med. Klin. — 2003. — 98. — 111-112.
15. Patsch J., Kohler T., Berzlanovic A., Muschitz C., Bieglmayr, Roschger P., Resch H., Pietschmann P. Trabecular bone microstructure and local gene expression in iliac crest biopsies of men with idiopathic osteoporosis // JBMR, accepted 2011.
16. Bordier P., Matrajt H., Miravet L., Hioco D. Mesure histologique de la masse et de la rйsorption des travйes osseuses // Pathol. Biol. — 1964. — 12. — 1238-1243.
17. Orwoll E., Ettinger M., Weiss S., Miller P., Kendler, Graham J., Adami S., Weber K., Lorenc R., Pietschmann P., Vandormael K., Lombardi A. Alendronate for the treatment of osteoporosis in men // N. Engl. J. Med. — 2000. — 343. — 604-10.
18. Orwoll E.S., Scheele W.H., Paul S., Adami S., Syversen U., Diez-Perez A., Kaufman J.M., Lancy A.D., Gaich G.A. The effect of teriparatide (human parathyroid hormone (1–34)) therapy on bone density in men with osteoporosis // J. Bone Miner. Res. — 2003. — 18. — 9-17.
19. Genant H.K., Wu C.Y., van Kuijk C., Nevitt M.C. Vertebral fracture assessment using a semiquantitative technique // J. Bone Min. Res. — 1993. — 8. — 1137-48.
20. Hahn M., Vogel M., Delling G. Undecalcified preparation of bone tissue: report of technical experience and development of new methods // Virchows Arch. а Pathol. Anat. — 1991. — 418. — 1-7.
21. Delling G., Werner M. Ist die histologische Untersuchung des Knochengewebes noch zeitgema Я? // Osteologie. — 2001. — 10. — 3-14.
22. Roschger P., Rinnerthaler S., Yates J., Rodan G.A., Fratzl P., Klaushofer K. Alendronate increases degree and uniformity of mineralization in cancellous bone and decreases the porosity in cortical bone of osteoporotic women // Bone. — 2001. — 29. — 185-91.
23. Roschger P., Gupta H.S., Berzlanovich A., Ittner G., Dempster D.W., Fratzl P., Cosman F., Parisien M., Lindsay R., Nieves J.W., Klaushofer K. Constant mineralization density distribution in cancellous human bone // Bone. — 2003. — 32. — 316-23.
24. Parfitt A.M., Drezner M.K., Glorieux F.H., Kanis J.A., Malluche H., Meunier P.J., Ott S.M., Recker R.R. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee // J. Bone Miner. Res. — 1987. — 2. — 595-610.
25. Rehman M.T., Hoyland J.A., Denton J., Freemont A.J. Age related histomorphometric changes in bone in normal British men and women // J. Clin. Pathol. — 1994. — 47. — 529-534.
26. Roschger P., Fratzl P., Eschberger J., Klaushofer K. Validation of quantitative backscattered electron imaging for the measurement of mineral density distribution in human bone biopsies // Bone. — 1998. — 23. — 319-326.
27. Roschger P., Paschalis E.P., Fratzl P., Klaushofer K. Bone mineralization density distribution in health and disease // Bone. — 2008. — 42. — 456-466.
28. Roschger P., Gupta H.S., Berzlanovich A., Ittner G., Dempster D.W., Fratzl P., Cosman F., Parisien M., Lindsay R., Nieves J.W., Klaushofer K. Constant mineralization density distribution in cancellous human bone // Bone. — 2003. — 32. — 316-32.
29. Fratzl-Zelman N., Roschger P., Misof B., Nawrot-Wawrzyniak K., Pцtter-Lang S., Muschitz C., Resch H., Klaushofer K., Zwettler E. Fragility fractures in men with idiopathic osteoporosis are associated with undermineralisation of the bone matrix. Accepted CTI 2011.
30. Hildebrand T., Laib A., Mьller R., Dequeker J. and Rьegsegger P. Direct three-dimensional morphometric analysis of human cancellous bone: microstructural data from spine, femur, iliac crest, and calcaneus // J. Bone Miner. Res. — 1999. — 14. — 1167-74.
31. Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction // Anal. Biochem. — 1987. — 162. — 156-919.
32. Kuliwaba J.S., Findlay D.M., Atkins G.J., Forwood M.R., Fazzalari N.L. Enhanced expression of osteocalcin mRNA in human osteoarthritic trabecular bone of the proximal femur is associated with decreased expression of interleukin-6 and interleukin-11 mRNA // J. Bone Miner. Res. — 2000. — 15. — 332-41.